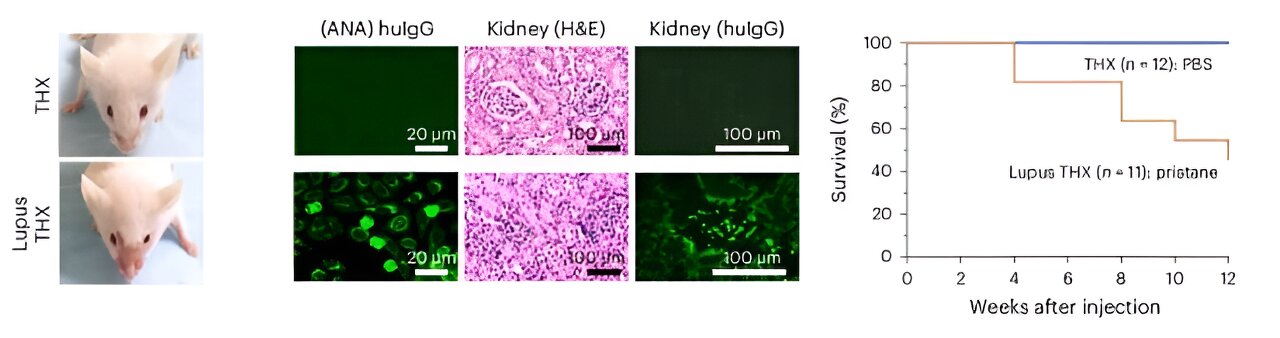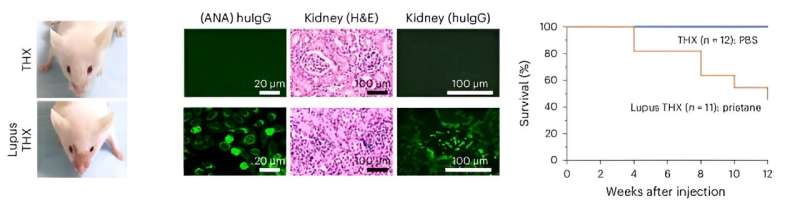
Left, Zygomatic rash in a (huNSGW41-derived) Lupus THX mouse (three weeks after pristane injection). Middle, Serum antinuclear IgGs (scale bar, 20 μm) and renal immunopathology (H&E and anti-huIgG immunofluorescence; scale bar, 100 μm) in Lupus THX and THX mice (12 weeks after pristane or PBS injection). Credit: Nature Immunology (2024). DOI file: 10.1038/s41590-024-01880-3
A breakthrough in biomedical research promises new insights into the development of immunotherapy and disease modeling. Scientists at the University of Texas Health Science Center at San Antonio have created a humanized mouse model with a human immune system and a human-like gut microbiome capable of mounting specific antibody responses.
The scientists were led by Paolo Casali, MD, University of Texas Ashbel Smith Professor and Distinguished Research Professor, Department of Microbiology, Immunology and Molecular Genetics in the Joe R. and Teresa Lozano Long School of Medicine. Casali has five decades of biomedical research experience in immunology and microbiology and is a leading investigator in the molecular genetics and epigenetics of the antibody response.
The aim of the multi-year project, which will be published in the August 2024 issue of Nature Immunologywas intended to overcome the limitations of currently available in vivo human models by creating a humanized mouse with a fully developed and functional human immune system.
Mice are widely used in biological and biomedical research because they are small and easy to handle, share many immune elements and biological properties with humans, and can be easily genetically modified.
However, many of the more than 1,600 immune response mouse genes are incongruent with their human equivalents, resulting in divergences or deficiencies of mice as predictors of human immune responses. This made the availability of a “humanized” mouse model that faithfully reproduces human immune responses a high priority.
The first humanized mice were created in the 1980s to model human HIV infection and the human immune response to HIV. Humanized mice were, and have been, created by injecting immunodeficient mice with human peripheral lymphocytes, hematopoietic stem cells, or other human cells.
However, previous and current models do not develop a fully functional human immune system, have a short lifespan, and do not produce efficient immune responses. This makes them unsuitable for the development of in vivo human immunotherapies, human disease modeling, or human vaccine development.
Casali’s team began by injecting immunodeficient NSG W41 mutant mice intracardially (left ventricle) with human stem cells that they purified from umbilical cord blood.
After a few weeks, once the graft is established, the mice are hormonally conditioned with 17β-estradiol (E2), the most potent and abundant form of estrogen in the body. Hormonal conditioning with estrogen was prompted by previous research by Casali and others, which showed that estrogen increases the survival of human stem cells, increases the differentiation of B lymphocytes, and increases the production of antibodies against viruses and bacteria.
The resulting humanized mice, called TruHuX (for truly human, or THX), have a fully developed and fully functional human immune system, including lymph nodes, germinal centers, human thymic epithelial cells, human T and B lymphocytes, memory B lymphocytes, and plasma cells that produce highly specific antibodies and autoantibodies identical to those of humans.
THX mice exhibit mature neutralizing antibody responses to Salmonella Typhimurium and SARS-CoV-2 virus Spike S1 RBD after vaccination with Salmonella flagellin and the Pfizer COVID-19 mRNA vaccine, respectively. THX mice are also susceptible to developing full-blown systemic lupus autoimmunity after injection with pristane, an oil that induces an inflammatory response.
Casali said the discovery of the THX mouse opens up possibilities for human in vivo experiments, for the development of immunotherapeutics such as cancer checkpoint inhibitors, the development of human bacterial and viral vaccines, and for modeling many human diseases. He also hopes the new approach could eliminate the need for nonhuman primates for immunological and microbiological biomedical research.
Because little research has been done on the influence of estrogen on the immune system, Casali hopes this discovery will spur further research into this topic.
“By critically harnessing the activity of estrogen to support human stem cell and human immune cell differentiation and antibody responses, THX mice provide a platform for human immune system research, human vaccine development, and therapeutic agent testing,” Casali said.
Using the THX model, the Casali lab is now investigating the in vivo human immune response to SARS-CoV-2 (COVID-19) at the systemic and local levels, and human memory B lymphocytes, the dependence on nuclear receptor RORα for their production, and the events leading to RORα expression and dysregulation.
They are also investigating epigenetic factors and mechanisms that mediate the production of human plasma cells, the cell factories that produce antibodies – literally thousands per second – against bacteria, viruses or cancer cells.
More information:
Daniel P. Chupp et al, A humanized mouse that elicits adult class-switched, hypermutated, and neutralizing antibody responses, Nature Immunology (2024). DOI file: 10.1038/s41590-024-01880-3
Provided by University of Texas Health Science Center at San Antonio
Quote: Scientists create first mouse model with complete, functional human immune system (2024, July 8) Retrieved July 9, 2024 from https://medicalxpress.com/news/2024-07-scientists-mouse-functional-human-immune.html
This document is subject to copyright. Except for fair dealing for private study or research, no part may be reproduced without written permission. The contents are supplied for information purposes only.